The interpretation of the effect of Guidelines for Submission of Clinical Trial Data on Drugs (Trial) upon the work of sponsors and data statistics companies
The interpretation of the impact of Guidelines for Submission of Clinical Trial Data on Drugs (Trial)
On July 20th, 2020, the CDE issued Guidelines for Submission of Clinical Trial Data on Drugs (Trial), which stipulated that the guidelines would take effect for chemical medicines and biological products from October 1st, 2020. The date of implementation of TCM are stipulated according to relevant regulations in the announcement of registration classification and application dossier of TCM issued by the NMPA. As the implementation date approaches, it is an important problem that needs to be solved urgently and encountered by each pharmaceutical enterprise that how to submit clinical data on drugs in a reasonable and compliant manners according to the guidelines.
At present, relevant staff of the CDE and many data statistics companies and have already interpreted relevant contents of the guidelines. The article will interpret the guiding significance and influence of the guidelines on the data submission strategy in the application of domestic pharmaceutical enterprises and the working process of data statistics companies.
1、CDISC standard will become the standard for clinical data analysis and data submission in China's pharmaceutical R&D industry.
1、CDISC standard will become the standard for clinical data analysis and data submission in China's pharmaceutical R&D industry.
The new guidelines propose that “sponsors are encouraged to submit application dossier related to the clinical trial data sets in accordance with Clinical Data Interchange Standards Consortium (CDISC) standard.” Meanwhile, the original database and the analysis database are given a clearer definition (that is to say: the original database usually contains raw data collected directly from case report forms (CRF) and external files, and may also contain very small amounts of derived data; to analyze database aims to count and analyze newly-established derivative database), thus correcting the misunderstanding that the original database can be directly exported and generated by EDC.
Founded in 1997, CDISC is dedicated to improving the efficiency of medical research and healthcare-related fields and promoting the sustainable development of public health, with more than 260 corporate membership worldwide. As a catalyst for industrial production and collaboration, CDISC organically combines the relevant areas of the healthcare industry and develops into a global, open and recognized standard for medical research data. SDTM and ADaM data standards CDISC formulates were mandated by FDA as of December 17, 2016, and PMDA as of March 31, 2020.
The new guidelines state that if the sponsor submits data in accordance with CDISC standard, the SDTM database may be regarded as the original database and the ADaM database as the analysis database, which logically indicates clearly that the SDTM and ADaM databases submitted to CDISC standards meet the requirements of the CDE statistical audit. The database submitted to other standards is still available, but whether the database meets the requirements of statistical audit needs sponsors’ own guarantee.
Therefore, from the perspective of risk assessment, it is the easiest method for sponsors to meet and reach the requirements and standards of the CDE review by adopting CDISC standard for submission of clinical trial data and by submitting SDTM and ADaM databases as the original clinical trial and analytical databases.
2、Data documentation and traceability of clinical trial data are given more clear requirements.
2、Data documentation and traceability of clinical trial data are given more clear requirements.
The new guidelines emphasize that “the original and analytical databases submitted must have corresponding data description documents”, and define the data description document as “one of the most important documents for regulators to accurately understand the content of the submitted data during review”. In addition, the CDE review “requires a good traceability between the data through the data description document (e.g., between the original data set and CRF or between analysis data set and the original data set), for the convenience of regulatory review.”
The guidelines specify that “a data description file is generally an Extensible Mark-up Language (XML) or Portable Document Format (PDF) file. If an XML data description file is submitted, a corresponding Extensible Stylesheet Language (XSL) file should be submitted together.”
The abovementioned requirements are generally tailored to define.xml in CDISC standards. Although the CDE does not mandate the use of define.XML as the standard for the submitted database description files, from the perspective of clinical data submission, the submission of corresponding defin.XML documents of SDTM and ADaM data sets as data description documents is the easiest strategy to meet the review requirements of the CDE in the absence of a uniform standard approved by the CDE for database description files.
3、The effect of the new guidelines on sponsors and data statistics companies
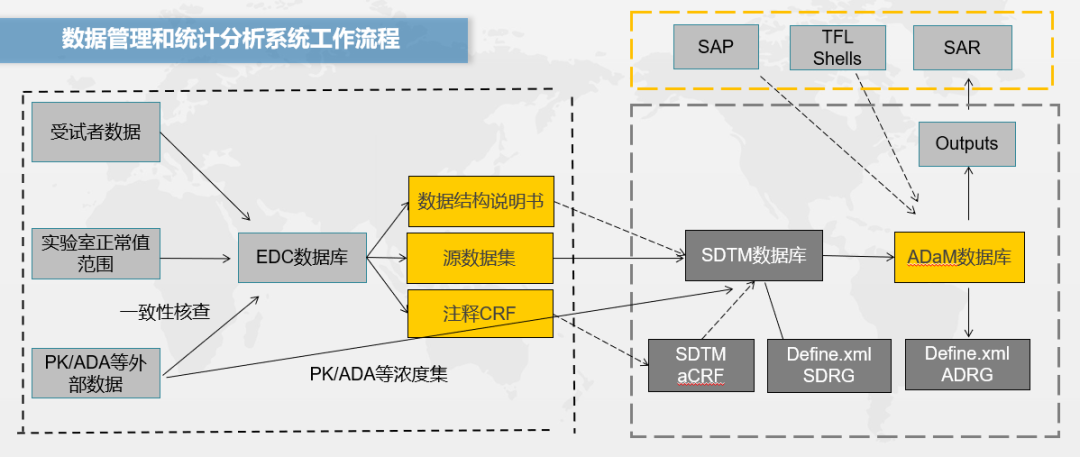
The release of the new guidelines has brought about huge changes in the workflow of data statistics companies. Data set exported by EDC is no longer suitable for the original database for clinical submission. The clinical information of EDC database needs to be integrated with external data to generate SDTM data set according to SDTM standard and also need to regenerate the annotated CRF (aCRF) according to SDTM standard.
 sponsors
sponsors
3、The effect of the new guidelines on sponsors and data statistics companies
data statistics companies
The release of the new guidelines has brought about huge changes in the workflow of data statistics companies. Data set exported by EDC is no longer suitable for the original database for clinical submission. The clinical information of EDC database needs to be integrated with external data to generate SDTM data set according to SDTM standard and also need to regenerate the annotated CRF (aCRF) according to SDTM standard.
The analysis data set (ADaM) is derived from SDTM data set and generates an additional description file defined. XML for SDTM and ADaM databases to meet the requirements of the CDE statistical review. SDTM aCRF, original database (SDTM database) and the corresponding description files of the original and analytical databases are additional work to meet the new guidelines.

The promulgation of the new guidelines has also had a significant effect on the projects to be submitted by the sponsors in the near future. The guidelines stipulate that chemical medicines and biological products shall be implemented from October 1st, 2020. That is, chemical medicines and biological products to be declared on October 1st, 2020 must be submitted in accordance with the new guidelines.
For clinical trials that are currently underway and cannot be completed before October 1st, 2020, additional budget is required to complete modification of the database and supplement of description files if the data set exported by EDC is submitted as the original data set according to the old guidelines during the period of data analysis design.
For example, by generating the original database in accordance with the new version of the guidelines, and rewriting the program code for analysis database generation and quality control, the analytical database is derived from the original database so as to meet the requirements of the CDE statistical review for database traceability, which is what is referred to in the new guidelines that “when submitting the database, the sponsors should ensure that the regulators can derive the analytical database consistent with the sponsors using the original database and can directly reproduce the results of statistical analysis consistent with the sponsors using the analytical database.”


